Pfizer-BioNTech COVID-19 Vaccine was NOT approved August 23, 2021
The News Release from the FDA on August 23, 2021 announced that the Pfizer-BioNTech COVID-19 Vaccine was approved. It was NOT! What was approved was a new product called Comirnaty.
They did not refer the application to the Vaccines and Related Biological Products Advisory Committee??
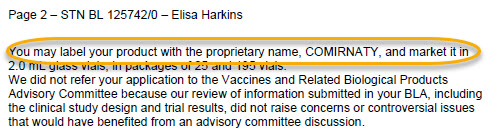
“We did not refer your application to the Vaccines and Related Biological Products Advisory Committee because our review of information submitted in your BLA, including the clinical study design and trial results, did not raise concerns or controversial issues that would have benefited from an advisory committee discussion.”
Why are they lying to us?
It is all about money and legal liabilities. It is also about them having protection from future trials based on the Nuremberg Code. The FDA states that there are legal differences between the Pfizer-BioNTech and Comirnaty COVID-19 Vaccines.
If the Pfizer Comirnaty product is the one that is licensed, why don’t we read much about it in news stories?
Most news stories since the end of August are about Pfizer-BioNTech COVID-19 Vaccine. Now the FDA has “approved” the Pfizer-BioNTech COVID-19 Vaccine for children 5-11 years old. Why are they still pushing the experimental product and not the approved Comirnaty product?
For Further Reading
- Major Law Firm Confirms FDA Deceived America With Its ‘Approval’ Of Pfizer Vax
- What You Need to Know About Comirnaty
- LEGAL HELP FOR RELIGIOUS EXEMPTIONS FROM VACCINATIONS
- Prevention & Early Outpatient Treatment Protocols for COVID-19
Subscribe For Updates
Subscribe to my mailing list and get updates to your email inbox.
Thank you for subscribing.
Something went wrong.

[…] can read more about what I refer to as the “FDA Shell Game“ on this website: Two Things Mainstream Media Didn’t Tell You About FDA’s Approval of […]